AstraZeneca AB v. Mylan Pharms. Inc.: Claim Construction of a Percentage Term Guided by the Written Description and Prosecution History
On December 8, 2021, the Federal Circuit in AstraZeneca AB v. Mylan Pharms. Inc.1 held that the claim construction of a percentage term should “‘most naturally align[] with the patent’s description of the invention,’ as further informed by the prosecution history.” 2 When posed with the question: “whether the concentration of [an excipient] being ‘0.001%’ means 0.001% within one significant figure—encompassing a concentration of [said excipient] in the range of 0.0005% to 0.0014%, . . . or it has a narrower meaning in view of the specification and the prosecution history—precisely 0.001% w/w [of said excipient] with only ‘minor variations,’” the Federal Circuit, in “a close call”, construed “‘0.001%’ as a precise number, with only minor variations[.]”3
AstraZeneca AB et al. (“AstraZeneca”) sued Mylan Pharmaceuticals Inc. et al. (“Mylan”) for infringement of all claims of U.S. Patent Nos. 7,759,328; 8,143,239; and 8,575,137 (collectively, the “asserted patents”).4 All of the asserted patents are listed in the Orange Book covering AstraZeneca’s Symbicort®, which is used for treating asthma and chronic obstructive pulmonary disease.5 Symbicort® consists of two active ingredients: “formoterol, a bronchodilator that opens the airway, and budesonide, a steroid that reduces inflammation in the lungs.”6 The inactive ingredients include PVP K25 (a formulation stabilizer) among other ingredients.7 Claim 13 of the ’328 patent is representative of the claims, which recites:
13. A pharmaceutical composition[] comprising formoterol fumarate dihydrate, budesonide,HFA227, PVP K25, and PEG-1000, wherein the formoterol fumarate dihydrate is present at a concentration of 0.09 mg/ml, the budesonide is present at a concentration of 2 mg/ml, the PVP K25 is present at a concentration of 0.001% w/w, and the PEG-1000 is present at a concentration of 0.3% w/w.8
The district court conducted a claim construction hearing concerning, inter alia, the construction of “0.001%,” the claimed concentration of PVP.9 “The district court construed ‘0.001%’ according to its ‘plain and ordinary meaning, that is, expressed with one significant digit.’”10 After claim construction, Mylan stipulated to infringement of certain claims of the asserted patents and the district court entered final judgment accordingly.11
Mylan also raised an invalidity defense against the asserted claims, but the district court in a bench trial determined that “Mylan failed to prove by clear and convincing evidence that the asserted claims would have been obvious in view of the prior art and entered a final judgment of no invalidity.”12 “The district court’s ultimate determination was based on several underlying factual findings, including a finding that one of the prior art references Mylan relied on in its obviousness combination, Rogueda,[13] taught away from the claimed invention.”14
After assessing the written description and prosecution history, the Federal Circuit held that:
Although the term “0.001%” without any broader context might indicate a range from 0.0005% to 0.0014%, here, in the context of the concentration of PVP, in light of the testing data in the specification and the amendments and arguments in the prosecution history, we conclude that the construction of this term most consistent with the intrinsic evidence is not so broad. Accordingly, we construe “0.001%” as that precise number, with only minor variations, i.e., 0.00095% to 0.00104%.15
The Federal Circuit acknowledged that “the term ‘0.001%,’ being expressed using only a single significant figure, would ordinarily, as an abstract number on a page, encompass a range from 0.0005% to 0.0014%” and under this standard scientific convention, “numbers falling within that range would typically be rounded up or down to 0.001%.”16 It, however, disagreed that “this ‘ordinary meaning’ controls absent lexicography or disclaimer” because “this narrow view of [the] precedent would necessitate adopting an acontextual construction of this disputed claim term, improperly isolating the numerical term from the more complete term ‘PVP K25 is present at a concentration of 0.001% w/w,’ as well as the specification and prosecution history descriptions of PVP concentrations.”17
With respect to the written description, the Federal Circuit stated that it dictates stability as “one of the most important factors which determines whether a compound or a mixture of compounds can be developed into a therapeutically useful pharmaceutical product.”18 “Specifically, the written description explains that ‘the concentration of PVP (0.001% w/w) used in this formulation has been found to give consistently stable formulations over the required dose range’” and it “repeatedly touts the superior stability of formulations with 0.001% w/w PVP.”19 Moreover, “the inventors tested formulations including PVP at concentrations of 0.0001%, 0.0005%, 0.001%, 0.01%, 0.03%, and 0.05% w/w and characterized each formulation for stability.” For instance, in Figure 3, “the formulation with 0.001% w/w PVP has a lower transmission measurement than the formulation with 0.0005% w/w PVP, meaning the formulation with 0.001% w/w PVP is more stable than the 0.0005% w/w PVP formulation”:
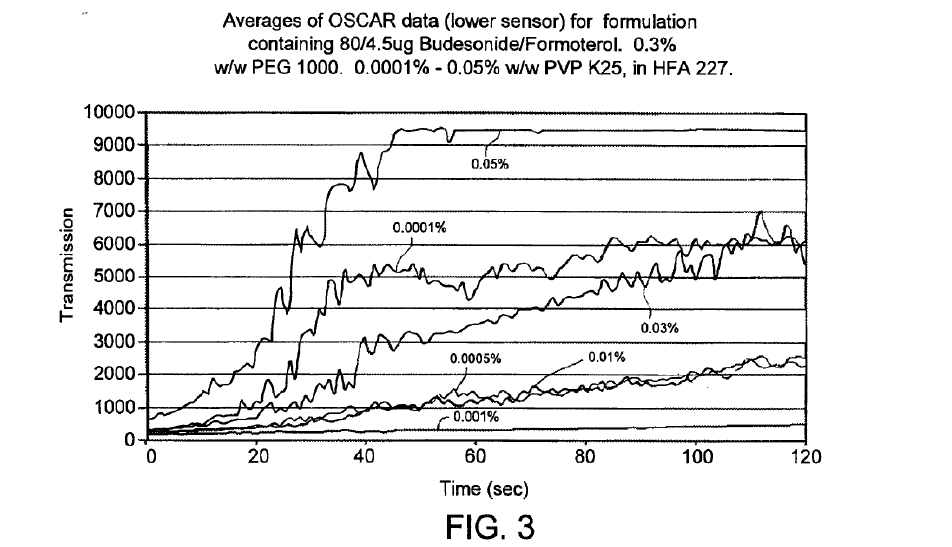
20. And more significantly in Figure 5, “the formulation comprising 0.0005% w/w PVP (second from the top) was one of the least stable formulations tested:”
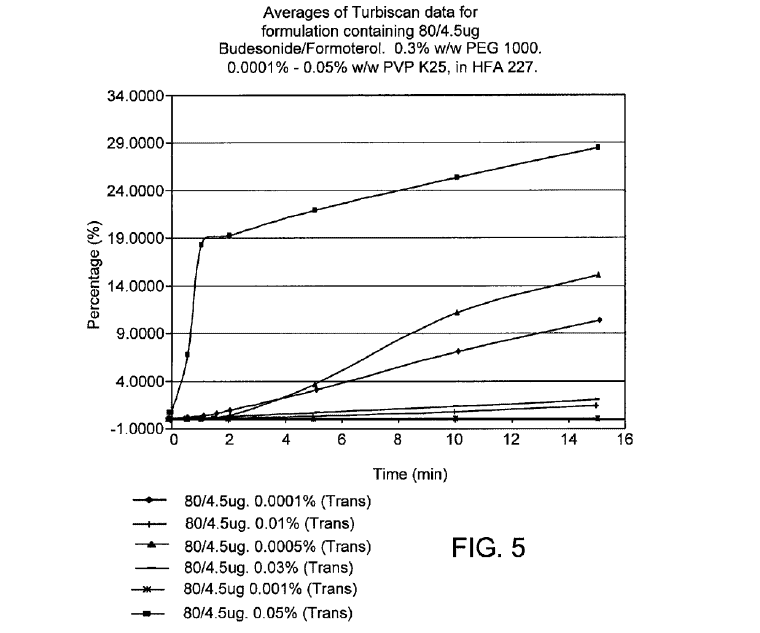
21
Accordingly, the Federal Circuit held that the written description makes it “clear that the inventors understood that a formulation comprising 0.001% w/w PVP is more stable than (and indeed, different from) a formulation with even a slight difference in the concentration of PVP, e.g., a formulation with 0.0005% w/w PVP” and “[t]his data leaves little room for doubt that slight differences in the concentration of PVP—down to the ten thousandth of a percentage (fourth decimal place)—matters for stability in the context of this invention.”22
As to the prosecution history, the Federal Circuit compared the original claims with claims that were ultimately allowed, and noted that the inventors upon the examiner’s rejections (1) limited the amount of PVP to 0.001% w/w without using the “about” qualifier that had been previously included in certain original claims, and (2) narrowed or cancelled previously presented “claims reciting a variety of different PVP concentration,” amending existing claims or introducing several new claims to recite a PVP concentration of “exactly” 0.001% w/w.23
Based on the above, the Federal Circuit adopted a “construction, which allows for only minor variations in the PVP concentration at the fourth decimal place, representing a 5% variation in the PVP concentration” and “more accurately reflects the level of exactness the inventors used in the written description in concluding that 0.001% w/w PVP is the most stable formulation, as well as the arguments and amendments in the prosecution history asserting that 0.001% w/w PVP is ‘critical’ compared to formulations with slightly more or less PVP.”24
Regarding Mylan’s invalidity challenge, the Federal Circuit upheld the district court’s finding that the prior art reference Rogueda taught away from the claimed invention and did not render the claims obvious.25 The district court compared certain control formulations in Rogueda—which Mylan argued rendered the asserted claims obvious—with Rogueda’s novel formulations.26 The district court found that the “novel formulations exhibited a ‘drastic’ reduction in the amount of drug adhesion compared to their controls” and that “the novel formulations had a narrower size distribution and smaller average particle size than the control formulations, noting that the particles in the novel formulations existed as ‘individual particles and not as clusters.’”27 Additionally, AstraZeneca’s expert testified that “a skilled artisan would consider the control formulations ‘just unsuitable,’ and therefore would not have any reason to use these control formulations as a basis for experimentation.” Based on the record, the district court concluded that a skilled artisan “would have been discouraged from incorporating the [control] formulations” because “the data cut against the very goal a skilled artisan would have been trying to achieve—a stable product with a consistent dose.”28 The Federal Circuit agreed and found no error in the district court’s conclusion that “Rogueda teaches away and does not render the claims obvious.”29
Judge Taranto dissented from “the majority’s holding that the term ‘0.001%’ should be construed as ‘that precise number, with only minor variations.’”30 He stated that “[i]n [his] view, ‘0.001%’ should be construed to have its significant-figure meaning, i.e., the interval 0.0005% to 0.0014%, as the district court held, with only one possible interval-shrinking change that cannot matter in this case.”31
First, Judge Taranto determined that the intrinsic evidence did not limit the scope of PVP concentrations to “a precise number with only minor variations.”32 He noted that the written description provides several formulations that were “considered excellent,” and formulations with “0.001%” w/w PVP “gave the best suspension ability overall[.]”33 He also explained that the prosecution history does not restrict the PVP concentration levels to “0.001%” with only minor variations because AstraZeneca neither represented nor conceded to such a narrow interpretation and its continuation applications further indicate that the PVP concentration levels are not so limited.34
Next, Judge Taranto criticized the construction of the term “minor variations” and stated that it adds “uncertainty” to the claim scope.35 He explained that the term effectively reinstates the “about” language that was removed in favor of the more precise “0.001%.”36 He clarified that the “withdrawal of [the] ‘about’ language does not imply that ‘0.001%’ was meant to have a non-interval meaning”; rather, “0.001%” was expressed as “a number having a well-defined interval (the ordinary significant-figure meaning), not one having the uncertain scope of ‘about’ or ‘minor variation.’”37
Finally, Judge Taranto noted that of the various PVP concentration levels that were tested and described in the written description and prosecution history, only “0.0005%” and “0.001%” have overlapping significant-figure intervals.38 He explained that although this overlap would result in an interval-shrinking change, such a change would exclude only those concentration levels that overlap between the significant-figure interval of “0.001%”—i.e., 0.0005% to 0.0014—and the significant-figure interval of “0.0005%”—i.e., 0.00045% to 0.00054%.39 He added that even if such an “exclusion were adopted, the language as construed would cover the interval 0.00055% to 0.0014%” effectively providing the same outcome as the district court’s decision.40 Accordingly, Judge Taranto would have affirmed the district court’s judgment.41
AstraZeneca AB v. Mylan Pharms. Inc underscores the importance of intrinsic evidence in claim construction—especially in the context of a percentage or range. In practice, this decision paves the way for future litigants to potentially argue during claim construction that, in certain instances, the written description and prosecution history trump the “ordinary meaning” within a standard convention even in the absence of lexicography or disclaimer.
777 South Flagler Drive
Phillips Point East Tower, Suite 1000
West Palm Beach, FL 33401